By Bonniemf
Incorporates work by NASA Earth Science Enterprise - Reworked by Bonniemf from the public domain file File:Carbon cycle-cute diagram.svg, CC BY-SA 3.0, Link
|
Phosphorus, like the other elements that move in biogeochemical cycles, is integral to how life took hold on Earth. It's thanks to phosphorus that our cells generate energy the way that they do. ATP (Adenosine Triphosphate) is a molecule that acts as an energy carrier for living things. It's held together with phosphorus bonds that release a sizable amount of energy when broken. It's this behavior that influenced multicellular life to form in the first place, and continues to be the way that life functions today!
In addition to being the pop caps on life's energy drinks, phosphorus is also the bridge responsible for double helix DNA similarly due to its bonding qualities. Beyond its molecular significance, it is also a component of cell membranes, the walls that protect cells. |
Phosphorus is a key ingredient in the molecule ATP, the energy carrier of cellular life. |
Phosphorus is also the reason DNA is shaped as a double helix. |
And if that wasn't enough, humans get one more perk from phosphorus. Our bodies do a lot of regulation and deal with a variety of acidic materials over the course of this regulation. One of the pieces of the human biology puzzle that keeps our bodies pH levels balanced is called the phosphate buffer system, which, you guessed it, functions thanks to phosphorus.
Even while researching this topic, I wasn't expecting to find this element putting in this much effort! Even beyond being an element integral in bonding with other elements necessary for carbon-based life, it goes above and beyond finding ways to be useful to the life it already supports. You go phosphorus! Where is PhosphorusSo far we've looked at how phosphorus accumulates itself in the biosphere, but how do we living things get this element in the first place?
|
|
While this is also an insignificant reservoir for phosphorus, trace amounts can also be found in the atmosphere when dust containing phosphorus gets dissolved into rainwater and sea spray. This is such a small fraction of the Earth's phosphorus, however, that the cycle primary factors in its movement between the lithosphere, hydrosphere, and biosphere. Speaking of...
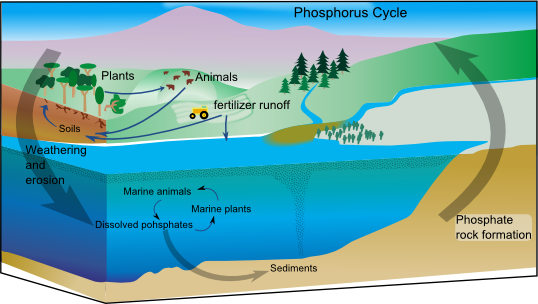
How Does Phosphorus MoveIf a majority of phosphorus is locked in the Earth's geology, how does it move? It's due to this characteristic that makes this cycle so slow, actually. Tectonic activity in the Earth's crust slowly pushes phosphorus-carrying rock, such as apatite, above the ocean's surface. After sufficient exposure to the Earth's elements, these rocks will break down and take hold in the soil. As the soil is churned by living organisms, some of this particulate phosphorus will rejoin the Earth's geology. When it doesn't, it's picked up by plants.
|
Phosphorus is the reason tooth enamel is the hardest biological substance on the Earth and is why steel utensils don't scratch your teeth. |
The phosphorus cycle moves with geologic time, taking thousands to millions of years to complete full cycles |
How Humanity Disturbs the CycleAs humanity uses resources found within the planet for uses beyond what the planet would naturally do on its own, we have to recognize that these activities have side effects. While it probably feels like the same song and dance between these pages detailing biogeochemical cycles, we have to understand the humans don't perform in a vacuum and any way we use resources on this planet could (and usually does) have a downstream effect. Quite literally as with the case of phosphorus.
Similar to nitrogen, the main way humans interact with the phosphorus cycle is by mining it for use in fertilizer. In fact, 80% of all mined phosphorus is used in creating fertilizers for our global agriculture industry. Though, simply mining and creating fertilizer with phosphorus isn't the core problem. It's what comes after the fertilizer is used. |
|
These oxygen-less freshwater and marine ecosystems form what are known as dead zones where no life can take hold. The surplus of nitrogen and phosphorus also acidifies the water, which can spread to adjacent ecosystems. Beyond death and destruction, turning these areas adjacent to our homes into aquatic wastelands has health risks to both us and our neighboring animals out of the water.
A potential silver lining with phosphorus is that it isn't as reactive as nitrogen. It primarily travels in particulate and dissolved forms, avoiding the atmosphere. In other words, the vectors for its movement are more limited, thus potentially more controllable. There have been a number of studies looking into how we can incorporate more wetlands adjacent to eutrophication zones to pull excess nitrogen and phosphorus out of the aquatic ecosystems and back into the soil. |
Eutrophication is the process by which a body of water becomes entrenched with nutrients, can cause rapid algae growth and decay, ultimately draining the water of all available oxygen |